\(\renewcommand\AA{\text{Å}}\)
Density¶
periodictable.density¶
The following properties are added:
- density, density_units (g·cm-3)
- Densities for solids and liquids are given as specific gravities at 20° C unless other wise indicated by density_caveat. Densities for gaseous elements are given for the liquids at their boiling points. Missing data are represented by None.
- density_caveat
- Comments on the density, if not taken in standard conditions.
- interatomic_distance, interatomic_distance_units (Å)
- Interatomic distance estimated from element density.
- number_density, number_density_units (cm-3)
- Number density estimated from mass and density.
Density for the isotope is computed assuming that the atomic spacing is the same as that for the element in the natural abundance.
>>> from periodictable import D, H
>>> print("H: %.4f, D: %.4f"%(H.density, D.density))
H: 0.0708, D: 0.1415
>>> print("%.14f" % ((D.density/H.density) / (D.mass/H.mass)))
1.00000000000000
The following plot shows density for all elements:
(Source code, png, hires.png, pdf)
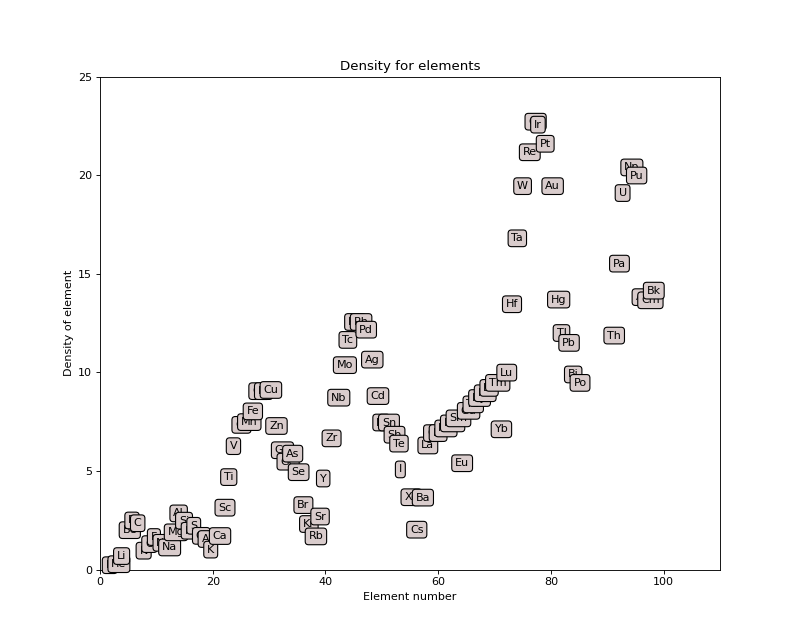
From the X-ray data book: http://xdb.lbl.gov/Section5/Sec_5-2.html
Data were taken mostly from [1]. These values are reproduced in [2].
| [1] | Lide. D. R., Ed., CRC Handbook of Chemistry and Physics, 80th ed. (CRC Press, Boca Raton, Florida, 1999) |
| [2] | The ILL Neutron Data Booklet, Second Edition. |
-
periodictable.density.density(iso_el)¶ Element density for natural abundance. For isotopes, return the equivalent density assuming identical inter-atomic spacing as the naturally occuring material.
Parameters: - iso_el : isotope or element
Name of the element or isotope.
Returns: density : float | g·cm-3
- Reference:
- ILL Neutron Data Booklet, original values from CRC Handbook of Chemistry and Physics, 80th ed. (1999).
-
periodictable.density.init(table, reload=False)¶
-
periodictable.density.interatomic_distance(element)¶ Estimated interatomic distance from atomic weight and density. The distance between isotopes is assumed to match that between atoms in the natural abundance.
Parameters: - element : Element
The element whose interatomic distance is to be calculated.
Returns: - distance : float | Å
Estimated interatomic distance.
Interatomic distance is computed using:
\[d = (m/(\rho_m N_A 10^{-24}))^{1/3}\]with units:
\[(\rm (g\cdot mol^{-1}) / ( (g\cdot cm^{-3}) (atoms\cdot mol^{-1}) (10^{-8} cm\cdot \AA^{-1})^3))^{1/3} = \AA\]
-
periodictable.density.number_density(element)¶ Estimate the number density from atomic weight and density. The density for isotopes is assumed to match that of between atoms in natural abundance.
Parameters: - element : element
Name of the element whose number density needs to be calculated.
Returns: - Nb : float | cm-3
Number density of a element.
Number density is computed using:
\[d = N_A \frac{\rho}{m}\]with units:
\[\rm (atoms\cdot mol^{-1}) (g\cdot cm^{-3}) / (g\cdot mol^{-1}) = atoms\cdot cm^{-3}\]