\(\renewcommand\AA{\text{Å}}\)
Basic usage¶
The periodic table is available on PyPI, and can be obtained simply with:
easy_install periodictable
This will install pyparsing if it is not already available. The numpy package must already be installed.
Access particular elements by name:
>>> from periodictable import hydrogen
>>> print("H mass %s %s"%(hydrogen.mass, hydrogen.mass_units))
H mass 1.00794 u
Access particular elements as symbols:
>>> from periodictable import H,B,Cu,Ni
>>> print("B absorption %s"%B.neutron.absorption)
B absorption 767.0
>>> print("Ni f1/f2 for Cu K-alpha X-rays f'=%.5f f''=%.5f"
... % Ni.xray.scattering_factors(wavelength=Cu.K_alpha))
Ni f1/f2 for Cu K-alpha X-rays f'=25.02293 f''=0.52493
Access isotopes using mass number subscripts:
>>> print("58-Ni vs 62-Ni scattering %s:%s"%(Ni[58].neutron.coherent, Ni[62].neutron.coherent))
58-Ni vs 62-Ni scattering 26.1:9.5
Access elements indirectly:
>>> import periodictable
>>> print("Cd density %.2f %s"%(periodictable.Cd.density, periodictable.Cd.density_units))
Cd density 8.65 g/cm^3
Import all elements:
>>> from periodictable import *
>>> print(periodictable.H)
H
>>> print(periodictable.H.mass)
1.00794
Deuterium and tritium are special isotopes named D and T some neutron information is available as ‘n’:
>>> print("D mass %s"%D.mass)
D mass 2.01410177784
>>> print("neutron mass %s"%n.mass)
neutron mass 1.00866491597
Process all the elements:
>>> import periodictable
>>> for el in periodictable.elements:
... print("%s %s"%(el.symbol,el.name))
n neutron
H hydrogen
He helium
...
Og oganesson
Another example for processing all elements:
>>> from periodictable import elements
>>> for el in elements:
... print("%s %s"%(el.symbol,el.number))
n 0
H 1
He 2
...
Process all the isotopes for an element:
>>> for iso in periodictable.H:
... print("%s %s"%(iso,iso.mass))
1-H 1.0078250319
D 2.01410177784
T 3.01604928132
4-H 4.02643
5-H 5.03531
6-H 6.04496
You can create a unique handle to an individual ion. In addition to storing the ion charge, this can be used to reference the underlying properties of the element or isotope:
>>> Ni58_2 = periodictable.Ni[58].ion[2]
>>> Ni_2 = periodictable.Ni.ion[2]
>>> print("charge for Ni2+ is %d"%Ni_2.charge)
charge for Ni2+ is 2
>>> print("mass for Ni[58] and for natural abundance: %.4f %.4f"%(Ni58_2.mass, Ni_2.mass))
mass for Ni[58] and for natural abundance: 57.9342 58.6923
The ion specific properties can be accessed from the ion using ion.charge for the ion index:
>>> import periodictable
>>> Fe_2 = periodictable.Fe.ion[2]
>>> print("[%.5f, %.5f, %.5f]"
... % tuple(Fe_2.magnetic_ff[Fe_2.charge].M_Q([0,0.1,0.2])))
[1.00000, 0.99935, 0.99741]
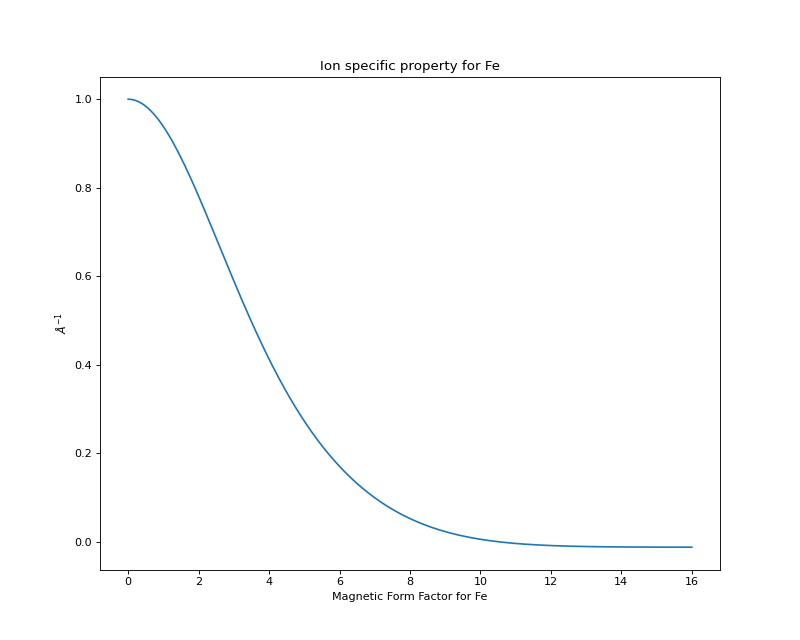
The following is a plot of the magnetic form factor vs. Q:
>>> import pylab
>>> Q = pylab.linspace(0,16,200)
>>> M = Fe_2.magnetic_ff[Fe_2.charge].j0_Q(Q)
>>> pylab.xlabel(r'Magnetic Form Factor for Fe')
>>> pylab.ylabel(r'$\AA^{-1}$')
>>> pylab.title('Ion specific property for Fe')
>>> pylab.plot(Q,M)
(Source code, png, hires.png, pdf)
Missing properties generally evaluate to None:
>>> print("Radon density %s"%periodictable.Rn.density)
Radon density None
Specific defined properties related to elements can be accessed in a table format as shown in following example :
>>> elements.list('symbol','K_alpha',format="%s K-alpha = %s")
Ne K-alpha = 14.6102
Na K-alpha = 11.9103
Mg K-alpha = 9.8902
Al K-alpha = 8.3402
...
Cf K-alpha = 0.1094
Es K-alpha = 0.1067
Fm K-alpha = 0.104